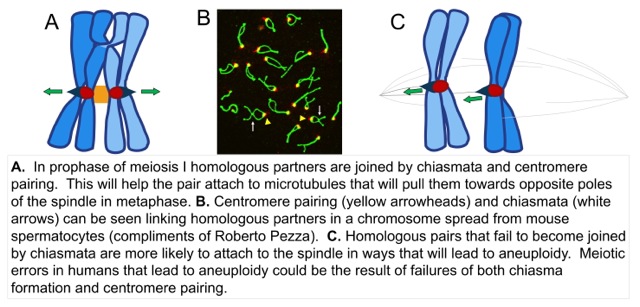
In both mitosis and meiosis, chromosome partners must attach to microtubules that emanate from opposite poles of the spindle – this is called bi-orientation. The microtubules anchor to a large protein structure called the kinetochore that is located at the centromere. Tension, created by pulling of the kinetochores towards opposite poles, stabilizes the microtubule attachments to kinetochores. This allows the chromosomes to remain attached until all the other chromosomes become attached. When all the chromosomes have become attached the partners are pulled to opposite poles of the spindle, a process called anaphase. In meiosis, the homologous chromosomes normally pair and become connected by a crossover and the tension is transmitted over this bridge. In meiosis there is a mechanism that pairs partner centromeres. We speculate that centromere pairing helps chromosome partners to become bi-oriented on the spindle in much the same way that crossovers do. This especially important when the partner chromosomes have failed to experience a crossover (all reviewed in our paper by Kurdzo). In collaboration with our neighbor, Roberto Pezza, we have found that centromere pairing can also occur in mammalian meiosis. Centromere pairing could have important human health implications as many human birth defects are caused when chromosome partners fail to recombine in meiosis, and partition improperly. This yields gametes with incorrect chromosome numbers. It may be that a centromere pairing process occurs in humans and normally acts to reduce the frequency of these errors. A major focus of our work is to investigate the mechanism of centromere pairing using yeast as a model system. Our goal is to determine how the centromeres become joined and how this joining promotes the proper attachment of the chromosome pair to spindle microtubules.
Kinetochore-microtubule interactions
![]() Correct segregation of chromosomes in both mitosis and meiosis depends on the development of correct attachments of the chromosomes to the microtubules of the spindle. In meiosis, this attachment process is especially tricky, because in the first meiotic division the sister chromatids segregate together and then are pulled apart in meiosis II. We are interested in how the structures of the kinetochores change thru meiosis (see Meyer et al., 2015) to allow for this special segregation pattern, and how the kinetochores attach and hold onto microtubules that depolymerize to pull the chromosomes toward the poles. A major focus has been the regulatory kinase Mps1. We have found that Mps1 has specialized meiotic roles that allow for sustained dragging of attached kinetochores across the meiosis I spindle (see Meyer et al., 2013). We are actively investigating the molecular basis by which Mps1 performs its critical meiotic functions. Much of this work is performed by constructing yeast strains with specific mutations, then monitoring chromosome behavior using live cell imaging methods. For example, in the image above we are following the movements of a chromosome, tagged at its centromere with green fluorescent protein, as it attempts to orient on the meiotic spindle. Images were captured every 45 seconds.
Correct segregation of chromosomes in both mitosis and meiosis depends on the development of correct attachments of the chromosomes to the microtubules of the spindle. In meiosis, this attachment process is especially tricky, because in the first meiotic division the sister chromatids segregate together and then are pulled apart in meiosis II. We are interested in how the structures of the kinetochores change thru meiosis (see Meyer et al., 2015) to allow for this special segregation pattern, and how the kinetochores attach and hold onto microtubules that depolymerize to pull the chromosomes toward the poles. A major focus has been the regulatory kinase Mps1. We have found that Mps1 has specialized meiotic roles that allow for sustained dragging of attached kinetochores across the meiosis I spindle (see Meyer et al., 2013). We are actively investigating the molecular basis by which Mps1 performs its critical meiotic functions. Much of this work is performed by constructing yeast strains with specific mutations, then monitoring chromosome behavior using live cell imaging methods. For example, in the image above we are following the movements of a chromosome, tagged at its centromere with green fluorescent protein, as it attempts to orient on the meiotic spindle. Images were captured every 45 seconds.
Mps1 and aneuploidy
Régis Meyer is leading a project to understand the relationship between Mps1, a protein that is a promising target for cancer therapy, and one of the most common features of cancer cells; aneuploidy. Aneuploidy is the result of losing or gaining chromosomes during cell division. Mps1 is a kinase that regulates the function of multiple proteins involved in cell division, and is critical to keep the correct number of chromosomes in each daughter cell. In most solid tumors, cells carry far more chromosomes than normal cells. An analysis of breast cancer cell lines revealed they had elevated chromosome numbers, high levels of Mps1 expression, and required high levels of Mps1 expression for their survival (Daniel et al., 2011). We speculate that in tumor cells with many additional chromosomes, the chromosome segregation machinery, and in particular, some process controlled by Mps1, is at its limits trying to align chromosomes on the spindle before cell division. Currently, it is not known which of the targets or processes under the control of Mps1 are the ones that are especially necessary for the survival of aneuploid cells. To address this, we are developing models using yeast cells to identify which phosphorylation targets of Mps1 are required for the survival of aneuploid cells.